Anyone who lives near the Gulf knows that salty sea air and humidity can wreak havoc on machinery. We tend to use the terms “corrosion” and “rust” interchangeably—but they’re not the same. So what’s the difference between corrosion and rust?
If you work in manufacturing, metal fabrication, or the automotive industry, you’ve seen what happens when metals and other materials begin to degrade. Often, parts that have endured harsh conditions for years can’t be saved and must be replaced. But the name of the damage you see depends on the materials that show wear.
Rust Is Corrosion, But Corrosion Isn’t Always Rust
Corrosion is the degradation of a substance that happens because of interaction with its environment. Corrosion is visible surface damage that happens when the molecular structure of a substance changes because of oxidation (a reaction with oxygen) or exposure to chemicals.
A variety of materials can display corrosion. It can happen to metal, but it can also happen to ceramics, polymers, wood, and even skin. That’s why workers who may encounter corrosive chemicals wear protective equipment. Corrosion can appear in a variety of colors, including greens and blues.
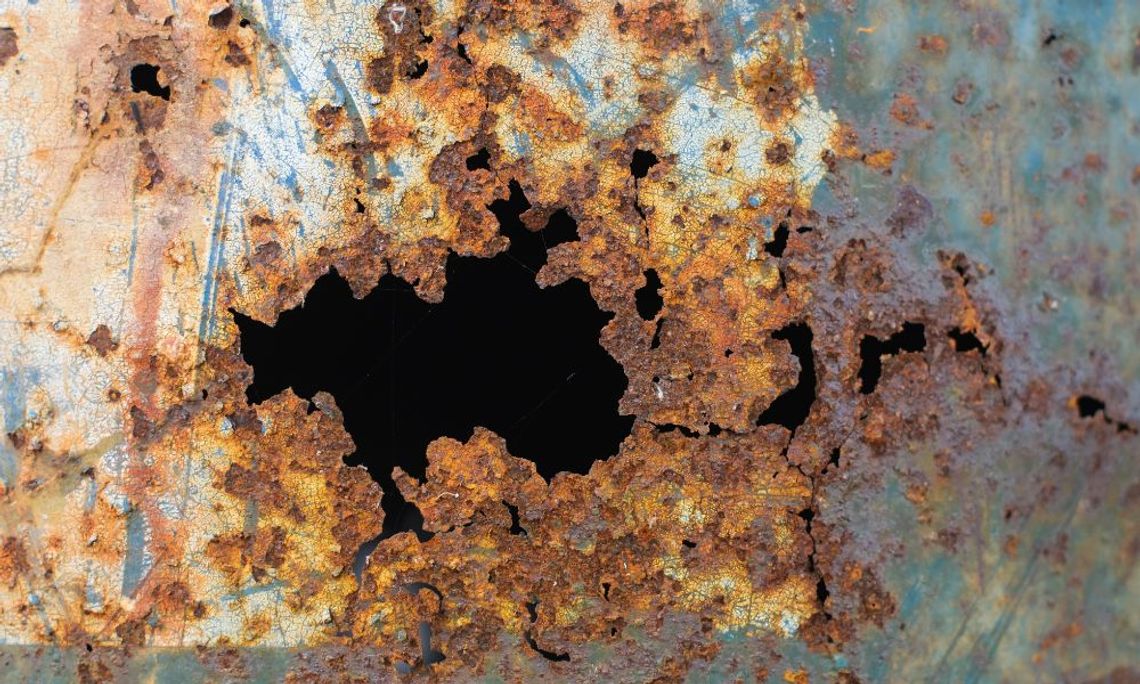
Rust is a type of corrosion, but it only happens in the presence of iron. When steel or cast iron encounters oxygen and moisture together, that orangey-looking, sometimes-flaky substance we call rust starts to appear. Iron molecules react with oxygen, releasing electrons that then flow through the metal and form hydrous iron oxide, or rust.
Types of Corrosion
Corrosion can occur across the entire surface of a substance. This “uniform corrosion” is easy to see. But corrosion can also occur in less visible ways. It could occur through pitting (the formation of a small corroded “cell”) that expands downward from the surface, turning a tiny dent into a large hole. Other forms of corrosion include cracking or corrosion in less visible crevices or contacts between metals, as with gaskets, bolts, or washers. Corrosion can also occur when two different types of metal contact each other in the presence of an electrolytic substance like salt water.
Preventing Corrosion
Corrosion and rust occur because of exposure to environments that contain chemicals, oxygen, and/or moisture that react with the material that corrodes. Applying a protective coating can prevent or at least slow down and hinder the chemical reaction that causes corrosion. Many industries use specialized types of protective coatings to preserve equipment, tools, and structures. Oil well drilling tools require protective coatings to shield them from corrosion due to extremes of temperature or exposure to harsh chemicals. Steel for construction or auto parts is painted with protective coatings to prevent rust.
When choosing a protective coating, it’s important to understand the difference between corrosion and rust. The materials you’re protecting will dictate what type of coating will be effective to prevent rust or other types of corrosion.

Comment
Comments